Abstract
Somatic TET2 mutations (TET2MT) are frequently found in myeloid neoplasia (MN) with a frequency that increases with age, consistent with the presence of subclinical TET2MT clones in asymptomatic older controls. Despite the prominent pathophysiologic role they play based on their prevalence; phenotype-genotype and prognostic associations have not been reliably established. We hypothesized that the diversity of TET2MT with regard to their configuration, genetic topography, and sub-clonal context, result in differences in biological, morphological, and clinical features.
We studied blood and bone marrow samples from 4930 patients with MN using deep sequencing for common myeloid somatic mutations, including TET2 . This analysis identified 1205 TET2MT patients with 1781 distinct TET2MT, as many had more than one TET2MT. The most common TET2MT were frame shift (47%) and nonsense (34%) mutations, both gene truncating events. Truncations were subdivided into those occurring upstream enough to effectively delete the whole gene (46%) and those that only led to deletion of the catalytic domain. TET2MT were found in 17% of patients with MDS, 46% with MDS/myeloproliferative neoplasm (MDS/MPN), 19% with MPN, 21% with pAML and 24% with sAML. Across all MN's, single mutations were more common than biallelic or homozygous, except in MDS/MPN, where there were similar proportions of single and multiple mutant cases (22% single vs . 24% biallelic/homozygous TET2).
TET2MT co-occurred most commonly with another TET2MT (43%), ASXL1 (21%), SRSF2 (18%), and NPM1 (13%). A similar distribution was seen in MDS, but with SF3B1 replacing NPM1 ; and in MDS/MPN, but with SF3B1 replaced by RUNX1 and CBL . In sAML the distribution of sub clonal lesions was similar to pAML, though with more mutations in NPM1 and FLT3ITD/TKD in pAML. Genes with the most significant differences in mutation rates between TETMTvs . TET2WT MDS/MPN patients were increased SRSF2 (34% vs . 15%, p<.001) and KRAS (8% vs . 3%, p<.001).
The rank of TET2MT within the clonal hierarchy is heterogeneous. TET2MT were found to be ancestral hits in 40% of mutant cases. A second TET2MT was the most common secondary hit following an ancestral TET2 hit. Other common 2nd hits included mutations in SRSF2, ASXL1, DNMT3A, and SF3B1 . TET2MTirrespective of ancestral or secondary configurations had no impact on survival outcome. However, when comparing TET2MT clonal size, TET2 allelic burden negatively affected survival (p=.016) for all patients with MNs.
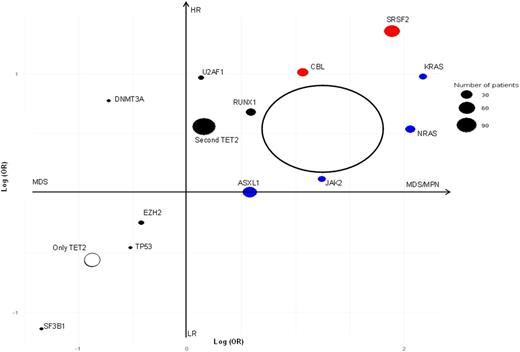
Having ascertained that the majority of TET2 lesions are ancestral hits, we set to determine their impact on progression, extent of dysplasia, and proliferation (monocytosis, increase megakaryocytes fibrosis). We used logistic regression to assess odds ratios (OR) of a gene being associated with MDS vs. MDS/MPN. We then determined univariate Cox regression hazard ratios for each gene and used these to construct 2-dimensional regression plots (Figure 1). This plot shows that TET2MT as a sole ancestral hit implies a greater likelihood of dysplasia and less of progression to high risk (HR) disease as compared to low risk (LR). Subsequent secondary hits change the features of the disease as shown in Figure 1. A second (biallelic) hit in TET2 increased the odds for the disease being myeloproliferative (OR=1.17), a secondary U2AF1 hit increased the odds of progression (OR=2.64), and a sub clonal SRSF2 mutation increased both (OR=6.61, 3.89). RAS family mutations had a moderate effect on progression (OR=2.66, 1.71), but induced proliferative features (OR=8.77, 7.80), whereas SF3B1 mutations associated with dysplasia and LR (OR=0.26, 0.32). In comparison, all combined TET2 hits (irrespective of the secondary context) were plotted as a circle (Figure 1).
In sum, our analyses demonstrate the genotypic/phenotypic impact of primary TET2 hits and how each subsequent mutation can affect the clinical phenotype of the disease.
Meggendorfer: MLL Munich Leukemia Laboratory: Employment. Nadarajah: MLL Munich Leukemia Laboratory: Employment. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Haferlach: MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal